Abstract
Introduction: Before the availability of SARS-CoV-2 mRNA vaccines, COVID-19 infection was responsible for up to 21/25% of deaths in allogeneic stem cell transplantation (Allo-SCT) recipients. This incidence was significantly reduced after 2 successive vaccines but the humoral response was shown to wane after a few months. The interest of one or two boosters has been confirmed thereafter, with a persistent and significantly reduced incidence of severe forms or deaths due to COVID-19 infection. Indeed, over a median follow-up of 6 months after a-third shot, we have recently reported long-term high protection of Allo-HSCT recipients (Chevallier, Hematol Oncol 2022). In this cohort, only 2 COVID-19 infections which did not require hospitalization occurred (1.4%) and only one patient died (0.7%). Since these latter results were obtained mainly during the Delta wave within the second semester of 2021, we have now considered the first semester of 2022 when the Omicron variant was predominant in France.
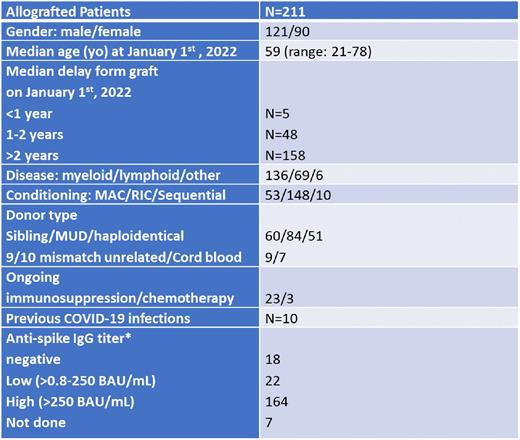
Methods: This monocentric observational study aimed at describing the incidence and severity of COVID-19 infections during the Omicron Wave (January 1st to June 30th 2022) in a cohort of 211 French allotransplanted patients (pts) who had been previously fully vaccinated (2 shots + 1 booster). Of them, 68 had also received a second booster between July 2021 and May 2022. The BNT162b2 anti-SARS-CoV-2 mRNA vaccine was used for all pts, except for 10 who received one shot of the mRNA-1273 vaccine. Some pts (n=12) had also received tixagevimab and cilgavimab (Evusheld) as preventive treatment after documentation of a negative serology or a low anti-spike IgG titer after 3 or 4 vaccines. Severity was defined by hospitalization and/or death related to a COVID-19 infection. Finally, predictive factors of infection were analyzed, including in particular the number of boosters received and the pts humoral status.
Results: Characteristics of the cohort are described in Table 1. During the 6 months considered, 37 (17.5%) pts were documented with COVID-19 infection. This was significantly higher compared to the incidence seen in our cohort during the previous period (17, 5% vs 2,1%, p<0.001), thus confirming the higher contagiosity of Omicron over the Delta variant. Almost 90% of these infections were mild since only 3 (1.14%) hospitalizations (including one in intensive care unit, ICU) and 1 death (0.47%) occurred. The pt hospitalized in ICU presented with a second mild COVID-19 infection 2.5 months after the first episode. Only 5 pts were within the first year post-transplant including 1 who developed a mild infection. The incidence of infection was significantly reduced in pts having received a second booster (8.8% vs 21.6%, p=0.03), although the hospitalization in ICU and the death were observed in this sub-group. Conversely, no influence on the rate of infection appeared related to a high anti-spike IgG titer (>250 BAU/mL) (15.8% vs 27.5%, p=0.13), a delay >2 years from the Allo-SCT (10.4% vs 19%, p=0.93) or an ongoing immunosuppressive/chemotherapy treatment (15.3% vs 18%, p=0.97). Interestingly, among the 10 patients who had contracted COVID-19 infection before January 2022, none showed reoccurrence of infection during the period considered.
Conclusion: During the Omicron wave in France, the incidence of COVID-19 infections has increased significantly in Allo-SCT recipients, although they were fully vaccinated (2+1 booster). However, almost 90% of these infections were mild and only 3 hospitalizations and one death were documented. This first indicates that anti-Spike mRNA vaccines remain efficient against Omicron variants. The significant protection against severe forms is likely related to a reactivation of memory immune responses upon infection. Indeed, after one year post-transplant, the majority of allotransplanted pts are well-protected after having been fully vaccinated (2 vaccines + 1 booster). However, a second booster seems to be even more protective and should be proposed to all allotransplanted patients.
Disclosures
Chevallier:Pfizer: Research Funding; Incyte: Research Funding; Abbvie: Honoraria; Jazz Pharmaceuticals: Honoraria; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal